Paris, Aix-en-Provence, December 3, 2019 – Affluent Medical is a French MedTech company specialized in innovative, minimally invasive implants designed to restore key physiological functions of patients suffering from structural heart and vascular diseases, as well as urinary incontinence. The company announces today the first patient implanted in its OPTIMISE II pivotal clinical study for KALIOS, its breakthrough adjustable mitral repair device.
Professor Martin Andreas, Principal Investigator of this pivotal study at the Vienna General Hospital (AKH), stated: “The KALIOS ring is easy to implant and adjustable at any time, during and post-surgery, and in different positions. KALIOS could be very effective in treating residual and recurrent mitral regurgitation. This is a long-awaited device, being an adjustable solution to each patient's need, that can make a significant difference in long-term outcomes compared to a conventional annuloplasty.”
Michel Finance, CEO of AFFLUENT MEDICAL, said: “The start of OPTIMISE II pivotal trial makes Affluent Medical now fully engaged in clinical validation for its structural heart programs. Kalios sets the ground for other major clinical trials coming soon. Pending regulatory approval on KALIOS, we are now potentially 2 years away from commercial stage.”
OPTIMISE II pivotal clinical trial: OPtimisation of surgical repair for Treating Insufficiency of the MItral Valve – Safety and Effectiveness evaluation.
OPTIMISE II is a pivotal, prospective, non-randomized, single-arm, multicentric and international study. It is expected to have approximately 62 patients implanted in up to 9 centers in 4 different countries across Europe (Italy, Austria, Germany and Switzerland). Patients enrollment is expected to be closed by Q2 2020, and primary endpoints are expected at the beginning of 2021 after a one-year patient follow-up.
Safety and effectiveness of KALIOS will be assessed as primary objectives, while effects of KALIOS device for the surgical treatment of mitral regurgitation on cardiac function and on patient functional status will be assessed as secondary objectives. These objectives will be followed at 30 days, 6 months and 12 months.
The present study was initiated following the OPTIMISE pilot clinical trial which was a prospective, non-randomized, single-arm (5 patients implanted), monocentric trial performed in Austria. It demonstrated positive results showing that the primary endpoint has been reached, proving the surgical safety of KALIOS.
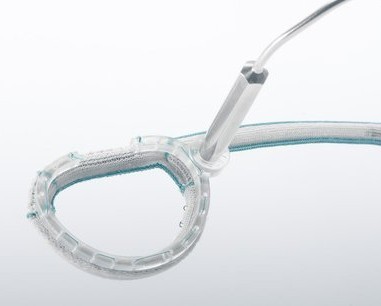
KALIOS, a breakthrough implant to be potentially launched in Europe by 2021
KALIOS can treat post-operative residual valve insufficiency, that can affect patient immediately after valve surgery, as well as the recurrent one, that can occur at a later stage after surgery. The size and shape of the implanted device can be adjusted percutaneously multiple times in the months/years following the surgery, offering patients a personalized long-term treatment.
Patients suffering from functional mitral regurgitation have today no mitral repair option that can address the recurrence, which is the progression of the disease post-surgery. Besides, the percentage of patients that receive suboptimal surgical treatment, meaning patients leaving the operating room with a residual mitral regurgitation or simply an insufficient valve coaptation, remains considerable. Both factors make the long-term outcomes of repair surgery unsatisfactory, which represents a need still unmet by the current structural heart therapies. KALIOS repair technology has the potential to fulfill this need.
The commercial launch of KALIOS in Europe is planned by the end of 2021, subject to the obtention of the authorization from regulatory authorities. It targets the global market of mitral valve repair and replacement devices, expected to reach $3.5 billion by 2022 and growing at a rate of 35% per year between 2017-2022, driven by the technological innovation of devices with percutaneous features.
About Affluent Medical
Affluent Medical is a French medtech company founded by Truffle Capital with the ambition to become a European leader in the treatment of heart and vascular diseases, which are the world’s leading cause of death, and of urinary incontinence, which today affects one in four adults. Affluent Medical is developing innovative, next-generation minimally invasive implants to restore key physiological functions in these areas. The company’s four major technologies are currently in preclinical and clinical phases, and a first medical device is expected to be launched by 2021. For more information: www.affluentmedical.com
Media contacts
Affluent Medical
Henri Lefebvre,
investor@affluentmedical.com
Tel. : +33 (0) 4 42 95 12 20
DGM Conseil
CFO thomasdeclimens@dgm-conseil.fr
quentin.hua@dgm-conseil.fr
Tel. : +33 (0)1 40 70 11 89